Multiple Hereditary Exostoses is an inherited disorder of bone growth. MHE can be referred to by various names such as Heredity Multiple Exostoses, Hereditary Multiple Osteochondromata, Multiple Carthaginous Exostoses, etc. People who have MHE grow exostoses, or bony bumps, on their bones which can vary in size, location and number depending on the individual. Although any bone can be affected, the long bones (legs, arms, fingers, toes), pelvis and shoulder blades are the most common, while the face and skull are generally unaffected. Boys and girls can both be affected. Older literature claimed that boys were more severely troubled by MHE, but bigger series of patients studied recently do not support this theory.
MHE is a condition that is passed by the genes of the affected parent to their children. It is called an ~autosomal dominant~ disorder which means that if one parent has the condition, chances are fifty percent that any child could also develop MHE. Occasionally, a patient will develop multiple exostoses with no previous family history of MHE. This situation is described as a spontaneous mutation meaning a genetic problem arose in that person without being inherited from a parent. Recently chromosomes (the packages that carry genes) 8, 11 and 19 have all been shown to be locations where the genetic information for MHE comes from. Some researchers feel there actually may be different types of MHE each caused by different genes at these locations.
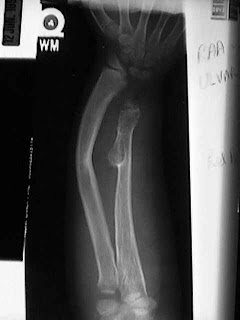
An exostosis is a bone growth that is abnormal or different from the underlying architecture of the bone. These "abnormal growths" are not cancer, They are benign. Sometimes doctors refer to exostoses as "tumors" which like exostose is a general term meaning abnormal growth. It is important to remember that not all "tumors" are cancer. Most tumors, like the exotoses of MHE, are benign. Exostoses start near the growth centers of bones which are near the ends of the bones, which is why bumps grow near the joints. They can be rounded or sharp and continue to grow while a child is growing. When a person is full grown, exostoses also stop growing.
MHE can be troublesome because the exostoses grow near the growth centers of the bone, they can make the growth center grow poorly, or only part of it grow poorly. This makes a lot of people with MHE somewhat shorter than average or have bowed arms or legs. Often, the forearm will bow out toward the lime finger, or the legs can become knock kneed. This is frequently concerning, but function is often normal though cosmetically, the bowing can be very troubling. Sometimes folks with MHE get stiff, especially in the elbows and hips, usually because their exostoses block some of their motion. While children are growing, exostoses can be painful. They seem to be very sensitive to getting bumped. Kids often develop exostoses on the inside of their knees and these can hit together when they run, which hurts!
Sometimes exostoses grow near nerves or tendons and press on them. In these cases, they often need to be removed so they won't damage the structure laying over them.
The most frightening complication of MHE is also one of the most uncommon. Rarely (less than 1% of the time), the benign exostoses of MHE can become a malignant tumor called chondrosarcoma. This happens almost always after adulthood when skeletal growth has ceased. Usually, patients who develop chondrosarcoma are in their 20's to 50's. If a person with MHE notices that an exostoses is getting bigger or painful after they have stopped growing they should get to their doctor! Growth and pain are two important warning signs that a benign tumor has become malignant. Chondrosarcoma is very rare, but it is something MHE families must know about.
According to this article, some people with MHE never require any treatment. They learn to compensate for deformity or decreased range of motion so they function normally. When deformity does occur, it often happens so slowly that the patient can compensate for it well, while others may require surgery to help them.
If an exostoses is painful, pressing on an important structure, cosmetically unattractive or if easily bumped, it can be surgically removed. Once removed, exostoses can reoccur (about 20 - 50% of the time), but may not regrow to a size large enough to be symptomatic. Removal itself is usually a fairly small procedure; some are removed without ever staying overnight in the hospital.
If an exostoses causes a growth abnormality, like bowing, sometimes just removing the exostoses early enough will allow the bone to straighten itself out and remodel as the child grows. Some bowing is so severe that not only must the exostoses be removed, but also the bone must be straightened. This can be done by either cutting the bone, straightening it and then holding it in place while it heals or if the child is still growing by changing the rate of growth on one side of the growth plate. Currently there are several options and your doctor should be able to explain them to you.
If an exostoses does become malignant and turn into a chondrosarcoma then it must be removed. A specialist in orthopedics and bone tumors would be required to help with this.
Scientists throughout tho world have demonstrated the genes for MHE are found on three different chromosomes. This leads to the belief that MHE is caused by at least three different genes with one or more on each chromosomes. It is known that the genes are located on chromosome numbers 8, 11 and 19 with number 8 being the most common location found.
Continuing research of the genes and how the proteins encode for them will give tremendous insight into the growth of cells. This information is important since MHE is a problem with the growth of cells.
Understanding the gene and the function of its protein might eventually provide the knowledge leading to actual treatment. The gene mapping studies will serve as the basis for the testing of children at risk for MHE. At the time of this publication, the availability of such testing is limited to a research setting. However, your physician could be equipped to perform this test in the near future. Information from this test could lead to the prevention of the development of exostoses and their complications.
Posted by:
Aini Syahida binti Mat Yassim
42101167
Reference
http://www.wheelessonline.com/images/med2.jpg
http://www.radix.net/~hogue/mhe.htm



